- Solutions
- All Solutions
- Consent
- Electronic Data Capture (EDC)
- EHR-to-EDC Integration | eSource
- Electronic Patient Reported Outcomes
- Randomization for Clinical Trials
- Recruit | Patient Recruitment Solution
- Reporting
- Share | Mobile Data Sharing for Research
- Services: Study Configuration | Migration | Integration | Training
- Oncology Clinical Trials
- Resources
- Blog
- Events
- About
- Contact
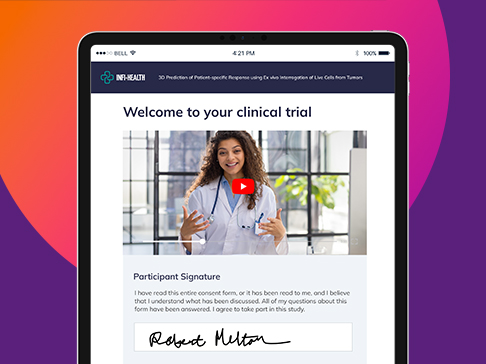
Go beyond the electronic-compliant signature. Deliver an engaging eConsent experience that simplifies, streamlines and automates electronic informed consent (eICF) for clinical trials.
Put Patients at the Center
Remove barriers for electronic consent management throughout clinical trial enrollment and participation. Meet participants wherever they are, on any device, with media-rich content to suit different learning styles. Nothing to download or install:
- Include images, audio and video in eICFs
- Verify comprehension with embedded questions
- Automate eICF notifications and reminders
- Support a multilingual experience

Overcome Informed Consent Challenges in Clinical Trials
Purpose-built solution to make informed consent easier, efficient and more engaging. eConsent is ready for:
- Multiple trial settings – DCT, remote, in-person and hybrid
- Global studies with multilingual populations
- Single or multiple signature needs
- Constant protocol changes and evolving informed consent requirements
Complete eConsent Platform for Streamlined Clinical Trials
Unified clinical workflows mean no more wasting time logging in and out of different systems. Seamless integration enables clinical trial study teams to easily see informed consent status in real-time. When protocols and requirements change, re-consenting workflows automatically trigger to help keep studies on track.


Data Privacy & Compliance
OpenClinica Consent delivers:
- Data privacy and security in compliance with HIPAA and GDPR
- Electronic signatures and audit logs in compliance with 21 CFR Part 11
- Automated delivery of signed digital copy to participant
- ISO 27001 and SOC2 security certification
-
 Convenient
ConvenientEasy for participants and study teams.
-
 Effective
EffectiveDeliver on the true purpose of electronic informed consent in clinical trials.
-
 Secure & Compliant
Secure & CompliantCompliant with HIPAA, GDPR, 21 CFR Part 11, ISO 27001, SOC 2.




