- Solutions
- All Solutions
- Consent
- Electronic Data Capture (EDC)
- EHR-to-EDC Integration | eSource
- Electronic Patient Reported Outcomes
- Randomization for Clinical Trials
- Recruit | Patient Recruitment Solution
- Reporting
- Share | Mobile Data Sharing for Research
- Services: Study Configuration | Migration | Integration | Training
- Oncology Clinical Trials
- Resources
- Blog
- Events
- About
- Contact
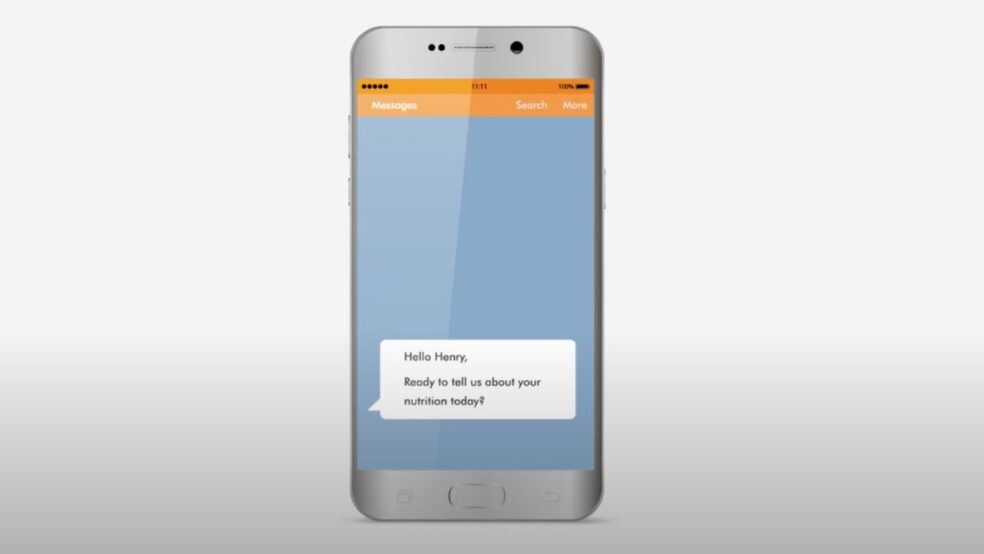
Patient engagement & retention is a top concern for clinical trial sponsors & sites. Improve both – and get higher quality data – with Participate™ electronic patient reported outcomes (ePRO). Participate delivers a fun and easy way for clinical trial patients to report crucial outcomes at their convenience. Just-in-time notifications help keep your study on track.
Get started with OpenClinica Participate (ePRO & eCOA)

Strengthen Patient Engagement
Make it super easy for clinical trial patients to stay engaged in your trial. Deploy an ePRO digital experience with images, video, visual analog scales and more–that works seamlessly on any device.
Zero Friction for Your Participants
Participate ePRO removes barriers for clinical trial participants. There’s no app to install and participants can use any device(s) they like (smartphones, tablets, desktops – you name it). Participants can securely access their dashboard without having to remember a username and password. Bring your own device (BYOD), no app to download, and no password to remember–that’s zero friction to boost engagement in clinical trials.


Fully Integrated ePRO Patient Engagement Solution
No more going in and out of different systems. Participate ePRO is fully integrated with OpenClinica’s EDC, so data managers and other study designers can enjoy creating the forms in one system and in one place. A single checkbox allows you to set either a clinician-based electronic case report form (eCRF) or a patient-facing ePRO form.
-
 Fully Integrated ePRO Clinical Data
Fully Integrated ePRO Clinical DataPatient-reported data instantly becomes part of your overall study data set.
-
 Clinical Data Quality and Security
Clinical Data Quality and SecurityWith Participate ePRO and OpenClinica’s powerful form capabilities, you increase data quality and speed of data acquisition.
-
 Compliant and Reportable ePRO
Compliant and Reportable ePROYour ePRO data is HIPAA compliant and fully auditable. A single audit trail clearly shows patient, clinician, and study team activity in a single view for easy clinical data analysis.
ePRO and clinical data,
together as one.
Patient-reported data instantly becomes part of your overall study data set.
Clinical data quality and security.
With Participate™ ePRO and OpenClinica’s powerful form capabilities, you increase data quality and speed of data acquisition.
Peace of mind.
And, your ePRO data is HIPAA compliant and fully auditable. A single audit trail clearly shows patient, clinician and study team activity in a single view.

Resources
Check out our free tools and thought leadership to learn more about clinical data management, electronic data capture, ePRO/eCOA, decentralized clinical research, and more!