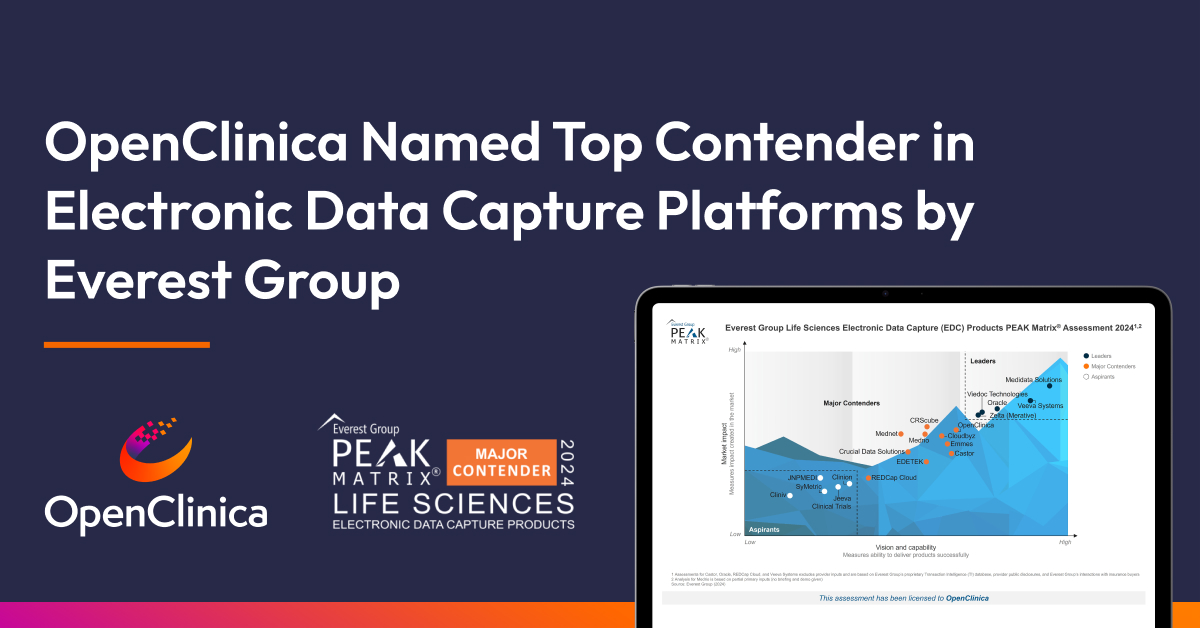
OpenClinica EDC earns high marks in technology capability, ease of deployment, and customer support
NEEDHAM, MASSACHUSETTS, UNITED STATES, September 25, 2024 /EINPresswire.com/
Everest Group, a leading global research and consulting firm, named OpenClinica a Major Contender in its Life Sciences Electronic Data Capture (EDC) Products PEAK Matrix® Assessment for 2024. The inaugural assessment evaluated 20 best-in-class EDCs based on the impact created in the market and the ability to deliver products successfully. OpenClinica differentiates itself from other EDCs with its vision and capability, earning high marks in technology capability, flexibility and ease of deployment, engagement and commercial model, and customer support. Nisarg Shah, Practice Director at Everest Group had this to say:
The EDC market has evolved significantly due to the increasing complexity of clinical trials, diverse real-world data sources, and the demand for efficient data management solutions, leading providers to develop comprehensive platforms that integrate data capture with advanced analytics and AI. There is also a clear trend towards enhancing user experience through low-code functionalities that allow workflow customization without extensive technical knowledge, addressing the need for real-time data insights and improved data accuracy,” says Shah.
OpenClinica offers a user-friendly EDC software that requires minimal programming knowledge, featuring single-click publish for mid-study changes, user management, data validation, and query management. OpenClinica’s strong partnership network includes CROs, hospital networks, industry consortia, and academic institutions, with clients appreciating its efficient support services and pricing tailored for their needs.
These strengths in ease of use, integration capabilities, and extensive partnerships have positioned OpenClinica as a Major Contender in the EDC PEAK Matrix Assessment 2024.”
— Nisarg Shah, Practice Director at Everest Group

OpenClinica’s EDC named the leading Major Contender in the 2024 Life Sciences Electronic Data Capture (EDC) Products PEAK Matrix® Assessment.
We’re honored that the Everest Group has recognized what our 500 clients already know,” said Ben Baumann, OpenClinica’s Co-Founder and COO. “OpenClinica’s EDC delivers self-service enablement, smart forms and data automation, helping to run 15,000 studies with three million participants, from small startups to large, complex platform trials.”
OpenClinica has continuously invested in its EDC and complementary product portfolio.  Earlier this year, the company debuted three major new features to its product suite and hundreds of enhancements to better meet the needs of its clinical trial customers. The three new features are state-of-the-art automated study calendaring, casebook enhancements, and single sign-on to seamlessly integrate user roles and permissions between OpenClinica solutions. OpenClinica’s study calendar infrastructure is a powerful rules engine and a robust, tested system that manages complex branching conditions and exceptions and, in turn, enables sponsors, sites and patients to adhere to even the most complicated clinical trial schedules.
Earlier this year, the company debuted three major new features to its product suite and hundreds of enhancements to better meet the needs of its clinical trial customers. The three new features are state-of-the-art automated study calendaring, casebook enhancements, and single sign-on to seamlessly integrate user roles and permissions between OpenClinica solutions. OpenClinica’s study calendar infrastructure is a powerful rules engine and a robust, tested system that manages complex branching conditions and exceptions and, in turn, enables sponsors, sites and patients to adhere to even the most complicated clinical trial schedules.
For a deep dive into OpenClinica’s EDC PEAK Matrix Assessment, download the report today.
ABOUT OPENCLINICA
OpenClinica accelerates clinical trials by automating data acquisition through its software-as-a-service platform. Offering a secure bridge between healthcare and research, OpenClinica is trusted by the world’s foremost life science companies, academic institutions, and government entities and has been used in more than 10,000 studies involving over five million patients. OpenClinica is proud to support hundreds of small, midsize and large research organizations spanning biotech, pharma, medical device manufacturing and contract research organizations. For more information, visit us at www.openclinica.com.
