- Solutions
- All Solutions
- Consent
- Electronic Data Capture (EDC)
- EHR-to-EDC Integration | eSource
- Electronic Patient Reported Outcomes
- Randomization for Clinical Trials
- Recruit | Patient Recruitment Solution
- Reporting
- Share | Mobile Data Sharing for Research
- Services: Study Configuration | Migration | Integration | Training
- Oncology Clinical Trials
- Resources
- Blog
- Events
- About
- Contact
Trusted by leading sponsors, CROs, and research institutions, OpenClinica’s electronic data capture (EDC) system accelerates clinical trial data management with automation, compliance, and scalability. Our advanced EDC technology simplifies data capture while ensuring accuracy, regulatory compliance, and seamless integration with existing workflows and technologies. With dynamic electronic case report forms (eCRFs) and real-time insights, OpenClinica helps you reduce risk, improve efficiency, and bring therapies to market faster—all while lowering operational burdens.
Core EDC: Value for All Stakeholders
Sponsors: Accelerate trials while ensuring compliance and data integrity.
- Stay informed with real-time study progress, reducing delays and inefficiencies.
- Improve trial outcomes with higher-quality data and automated compliance tracking.
- Save time and money with fewer data entry errors, faster site onboarding, and streamlined workflows.
- Rest easy knowing your data is secure with enterprise-grade protection and full regulatory compliance.


Sites: Keep sites productive, compliant and engaged.
- Provide sites with an easy-to-use platform that ensures accurate, timely data entry.
- Automate workflows with notifications for pending tasks, required actions, and study milestones.
- Streamline site management with auto-managed study calendars and built-in electronic signatures.
Participants: Frictionless data capture, better engagement.
- Allow participants to securely provide data anytime, anywhere from their own device.
- Automate reminders and notifications, reducing missed entries and improving compliance.
- Simplify the participant experience, ensuring seamless data collection without added burden.


Data Managers: Maintain full control over study data—without the hassle.
- Easily build, preview and publish studies with a collaborative, drag-and-drop interface.
- Provision sites and users effortlessly, ensuring the right access for every role.
- Quickly resolve queries and export datasets in a structured, regulatory-compliant format.
Monitors: Simplify oversight with real-time, mobile-friendly tools.
- Give monitors a centralized dashboard to track tasks and site activities at a glance.
- Enable on-the-go monitoring, making source data verification and compliance checks easier.
- Ensure faster issue resolution with a clear view of queries and site performance.

Ready to see how Core EDC can make your trial easier?
Key EDC Features
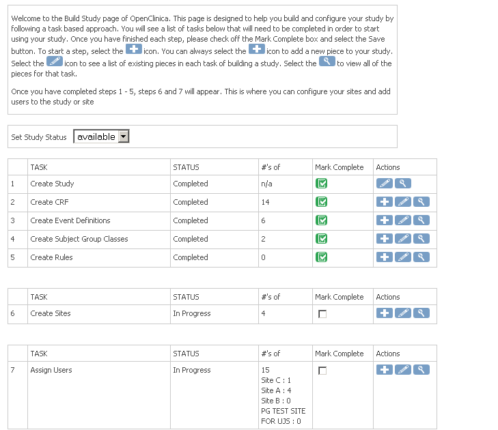
Build
Design and manage study workflows effortlessly with a powerful, intuitive interface.- Define and adjust events and forms in real time using a no-code study builder.
- Seamlessly collaborate with your team in a centralized system, with audit logs ensuring full compliance and version control.
- Update forms anytime while the system automatically tracks version history to maintain data integrity.
- Preview forms exactly as users will see them, ensuring a flawless experience before deployment.
- Launch studies in minutes, with the ability to publish to test or production environments instantly.
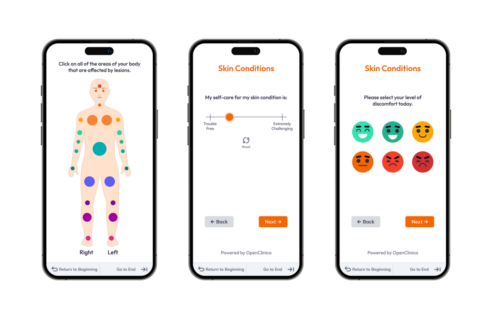
Capture
Streamline data collection with a flexible, user-friendly experience.- Eliminate manual effort with mobile-friendly, real-time validated forms featuring edit checks and auto-save.
- Upload bulk data efficiently through simple imports or seamless API integrations with your existing systems.
- Enable direct participant data capture on any device, improving engagement and compliance.
- Built-in randomization within eCRFs—no extra tools or manual steps needed.
- Securely invite study managers and sites, with customizable permission controls.
- Effortless site and user management—create once, and use across multiple studies
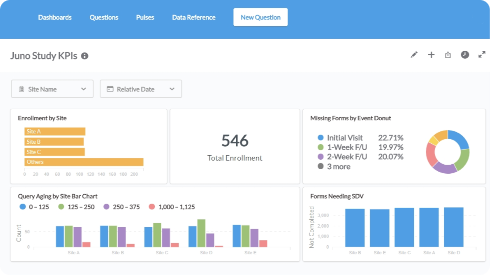
Report
Gain real-time insights and drive smarter decisions.- Access operational and clinical data instantly to proactively manage queries, trends, and adverse events.
- Track site activity & performance in real time, monitoring enrollment by site, data entry progress, query resolution times, and overall compliance.
- Visualize key metrics with dynamic bar charts, line graphs, and custom dashboards.
- Automate report distribution, sending updates to team leads and sites on a scheduled or event-triggered basis
An EDC Built for Growth
-
 Optimized Cloud
Optimized CloudMaximize the performance and reliability of your studies with stellar support, zero data loss, and disaster recovery. Rest assured with our validated and secure global infrastructure. SOC, ISO27001 certified.
-
 Training and Support
Training and SupportExpert guidance, hands-on training, and rapid support to keep your studies running smoothly. Learn to build and manage studies with confidence.
-
 Regulatory Compliance
Regulatory ComplianceBuilt-in compliance with GCP, 21 CFR Part 11, and global regulations. Complete validation documentation and audit support ensure you stay audit-ready.
-
 Cost Effective
Cost EffectiveScalable, budget-friendly EDC that grows with you. Get enterprise-grade capabilities without enterprise-level costs.