 Your study database has just locked. To celebrate, you decide to treat your two in-house monitors to dinner in the city. You’d like to offer your colleagues a choice of three restaurants. Take a moment and imagine which three restaurants you’d choose. Got it? Now suppose you recall that one of your monitors follows a gluten-free diet. Does that change your selection? If all of your initial picks specialized in wheat pasta, it ought to.
Your study database has just locked. To celebrate, you decide to treat your two in-house monitors to dinner in the city. You’d like to offer your colleagues a choice of three restaurants. Take a moment and imagine which three restaurants you’d choose. Got it? Now suppose you recall that one of your monitors follows a gluten-free diet. Does that change your selection? If all of your initial picks specialized in wheat pasta, it ought to.
What’s good for dinner plans is essential for study conduct, when data quality and safety are on the line. An unremarkable value for height in one participant ought to trigger a query for another; for example, for a teen and a six-year-old enrolled in a pediatric trial. To evaluate the input in one field based on data in another, data managers rely on cross-field edit checks. If the fields are part of distinct forms, that evaluation is known as a cross-form edit check.
At OpenClinica, we extend this capability. We give data managers a tool to make their forms responsive to any element they choose from their database, from the participant’s most recently recorded blood pressure to the start date of the last dosing visit. Using easy-to-understand syntax in the form definition, data managers may reference any item in the database to trigger dynamic edit checks, make calculations, show/hide relevant information, and even change the content and logic of the active form. Your forms can now “know it all” to present a fully contextualized data capture experience.
We developed this feature to help our users:
- capture more consistent, higher quality data,
- drive protocol compliance,
- highlight potential safety issues, and
- mitigate the risk of unnecessary study procedures.
The capability goes far beyond cross-form edit checks. This is total study intelligence. Below is the first of three case studies we’ll present this month that illustrate the difference. If your study has requirements that resemble these, don’t hesitate to contact us for a more in-depth guided tour.
Case #1: Age- and Sex-Dependent Normal Lab Range
One person’s high blood pressure is another person’s normal. Why? The primary reasons are differences in age and sex. When it comes to lab results, “normal” may also be a function of the specific lab conducting the analysis. But not all studies rely on lab-specific ranges. For clarity’s sake, let’s imagine a study that will evaluate lab values against standards that are lab-independent. Known as “textbook ranges”, these ranges define the upper and lower bound of normal based solely on patient-specific factors. Below is a table indicating the lower and upper limits of normal for DHEA-S in adult males and females:
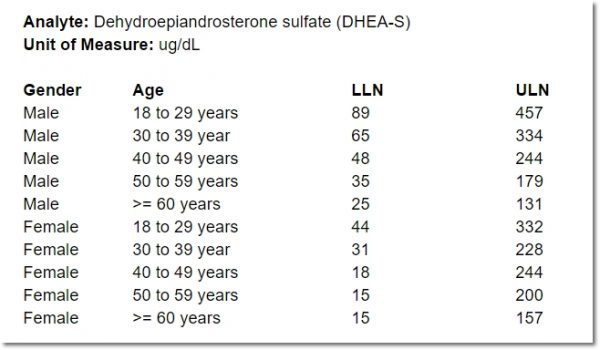
Chances are that a form associated with a screening event has already captured a participant’s sex and date of birth. A subsequent event may include a lab form. It’s inefficient to ask for the participant’s sex and age on this lab form. Sex has already been documented, after all, and age requires a calculation that compares the specimen collection date with the participant’s date of birth. Asking a CRC to make that calculation opens the door to error, especially if collection occurred close to the participant’s birthday. But age and sex information is required to determine whether an entered value is above or below the limits of normal.
Enter cross-form logic, which handily “pulls in” the required data from an external form. In the current case, an expression within the form compares the specimen collection date with the externally-supplied date of birth, to calculate participant age. That age, together with the externally-supplied sex and lower and upper limits indicated within the lab form, are all it takes to instantly evaluate the lab value against the appropriate range for the participant. Results of that evaluation may trigger or hide additional fields, get piped into question or response text for a separate item, or simply provide instructions, as shown in this video.
