ICYMI, OpenClinica recently debuted market-leading automated study calendaring functionality.
Our study calendaring capabilities automate scheduling and protocol visits and enable automated email and text notifications to study teams and participants. Automatic scheduling and closing of visit events can be triggered based on visit status, time windows or participant creation. The powerful study calendar logic engine supports rich conditional logic and is particularly aligned to the needs of complex protocols in areas such as oncology clinical trials and direct data capture studies.
Automated Study Calendaring for Clinical Trials: State-of-the-Art & Three Years in the Making
What you may not know is that three years of effort went into building OpenClinica’s study calendar infrastructure.
That’s right. Three years to build our incredibly powerful rules engine. Imagine constructing an intricate, state-of-the-art skyscraper. Each floor represents a critical component, each beam meticulously placed to support the grand structure. The anticipation, expectations, excitement, stress and readiness throughout this process parallel our journey in developing our study calendaring system.
At OC24, our annual global customer conference, I had the opportunity to take a deeper dive into the richness of OpenClinica’s study calendaring. In short, our clinical trials study calendaring – especially when combined with OpenClinica eConsent, Participate, Unite, Randomize and Share –
- Facilitates extremely high levels of user engagement,
- Increases the timeliness of data,
- Minimizes deviations, and
- Creates a better experience for sponsors, sites and patients.
What’s more is that our state-of-the-art study calendaring simplifies clinical trials in a way that makes it easier to run virtual, digital, hybrid and decentralized clinical trials.
Study Calendaring for Clinical Trials: Time-Saving Triumph
OpenClinica’s protocol automation through participant calendars is a robust, tested system that manages complex branching conditions and exceptions and, in turn, enables sponsors, sites and patients to adhere to even the most complicated clinical trial schedules.
Some of the features I highlighted for OC24 attendees are:
- Automatic scheduling and closing of visit events can be triggered on visit status, time windows or participant creation.
- Visit windows for auto-scheduling and auto-closing events.
- Time-specific actions will respect the participant-specific, site time zone configuration.
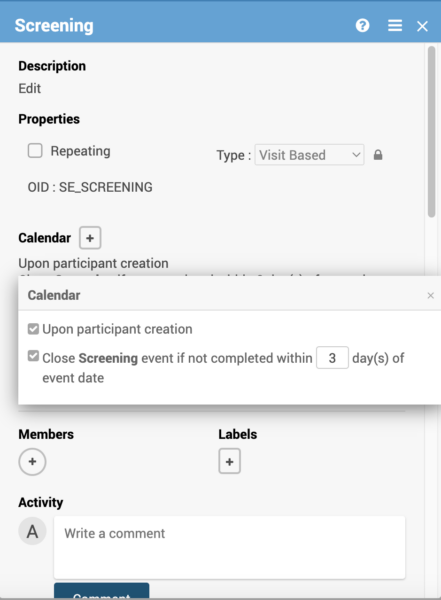
Creating a calendar event action for the first event in your study allows you to schedule that event based on that participant creation.
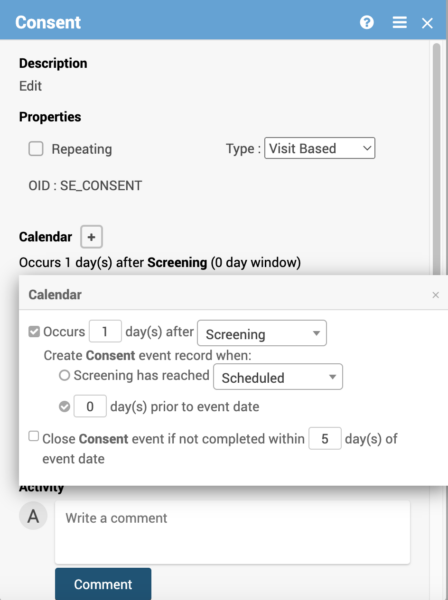
Data managers can define visit windows for auto-scheduling and auto-closing events. OpenClinica’s study calendaring for clinical trials enables the creation of time-saving automations through the drag-and-drop study design
OpenClinica’s brand-new dynamic rules engine adds:
- Additional power,
- Better management tools, and
- Integrated capabilities into the drag-and-drop, point-and-click study design environment.
In other words, it’s an extraordinary leap forward in scheduling and rescheduling critical study events. We empower you to:
- Define email and SMS notifications for non-repeating visit-based events,
- Send notifications to participants or any predefined email addresses,
- Send upon event creation or completion as well as before or after the event’s start date,
- Set up multiple notifications for an event, each configured to be sent at a specific time of day that aligns to the participant-specific time zone configuration.
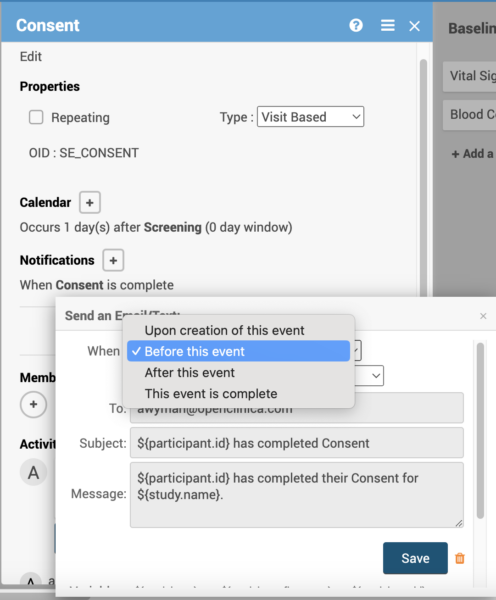
Define when you want to send a notification.
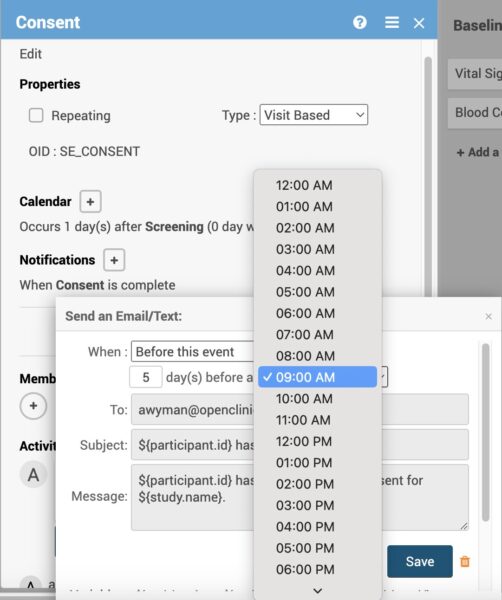
Based on when the notification meant to be sent, you can select a time to send it. This is based on each participant’s site timezone. OpenClinica’s study calendaring for clinical trials enables the right notifications at the right time, a time-saving triumph!
Beyond what you can directly do today, there is an incredibly powerful logic engine that supports complex conditional expressions. That means studies can trigger events based on data in the participant’s clinical record, administrative or logistical information captured in forms, and more.
The advanced capabilities of OpenClinica’s study calendaring include:
- Show/hide forms within an event-based rule logic,
- Logic can be based on item data values,
- Support repeating events, and
- Familiar Xpath logic that is the same as forms.
To say that I’m excited about OpenClinica’s study calendaring today is only dwarfed by what’s coming next.

