Once again, a blog about electronic case report forms (eCRFs) tops the list of the most read blogs of 2024, by a wide margin. As eCRFs are the foundation for successful studies, it’s no surprise that our readers continue to find this content useful. The most-read blog of 2024 explains the components of eCRFs – a digital, usually web-based, questionnaire for collecting data about a study participant – terminology such as form label, group label, item label, item hint or description, field, value, choice label and data validation/edit check.
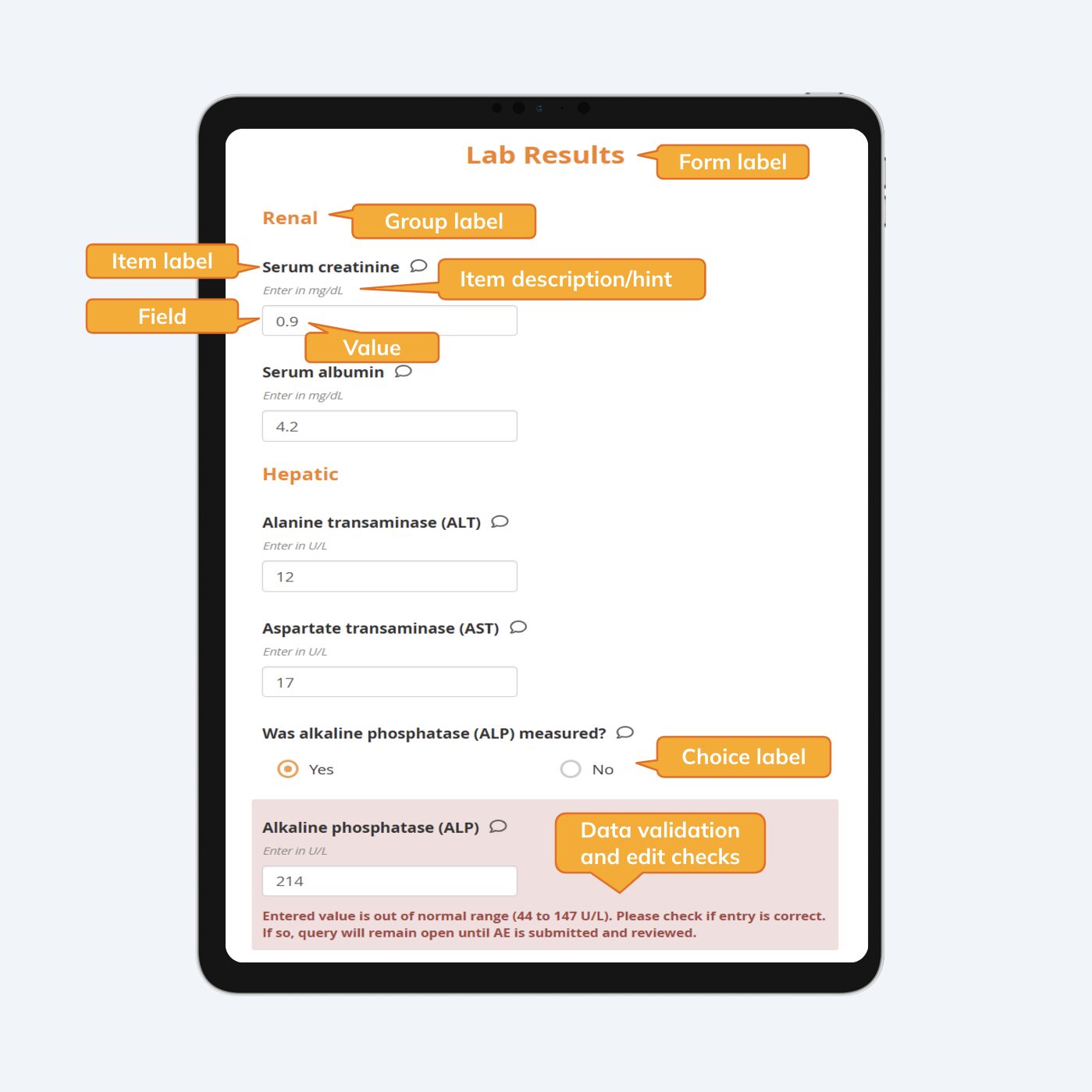
The components and terminology used when designing electronic case report forms (eCRFs)
A number of other top blogs of 2024 are educational content such as Registry Studies Are Unique: 8 Distinct Features, Eight Ways Medical Device Clinical Trials from Drug Trials and One Easy Way to Reduce Data Errors and CRC Burnout, a blog that details three examples of how to use cross-form logic to simplify the job of clinical research coordinators.
Another reader favorite is a blog about using decentralized clinical trials to improve patient engagement. This most-read blog discusses six ways to create a frictionless experience for patients, including an ultra-reliable user experience and automated notification rules. To read this DCT blog, Decentralized Clinical Trials Improve Patient Engagement, click here.
A popular topic with the OpenClinica blog authors and our blog readers is EHR-to-EDC eSource. Believe it or not, an especially popular blog links Taylor Swift’s Eras Tour and EHR-EDC eSource integration.
Another top 10 blog details how EHR-to-EDC integration saves time and money. For example, an adaptive platform trial of repurposed therapeutics for patients hospitalized with a severe infectious disease used eSource technology to achieve time savings. The use of EHR-EDC eSource delivered:
- A 16-minute per form savings for elapsed data entry time on the primary daily eCRF, a 61 percent reduction compared to manual entry;
- For just 52 patients studied at one site (UCSF) with a 15-day treatment course, 208 hours of clinical research coordinator time was saved;
- The elimination of data errors by the automated, validated EHR-loaded data and the downstream costs associated with cleaning, source data verification (SDV) and validation;
- Low implementation costs, and
- Reusability across sites.
Rounding out the top ten, most read blogs are about mid-study data migrations and direct data capture.
The goal of the OpenClinica blog is to create content that is useful and educational to our readers. If there are topics that interest you, please reach out to Mary Lou McCoy at mmccoy@openclinica.com
2024 Staff Favorites Include:





