On November 30, 2022, ChatGPT was released for public use. A mere five days later, there were one million users. Fast forward to summer 2023, ChatGPT is available in more than 30 countries, its user base is more than 100+ million users – roughly 15 percent of which are Americans – and the website of the company that created ChatGPT, openai.com, receives one billion visits per month.
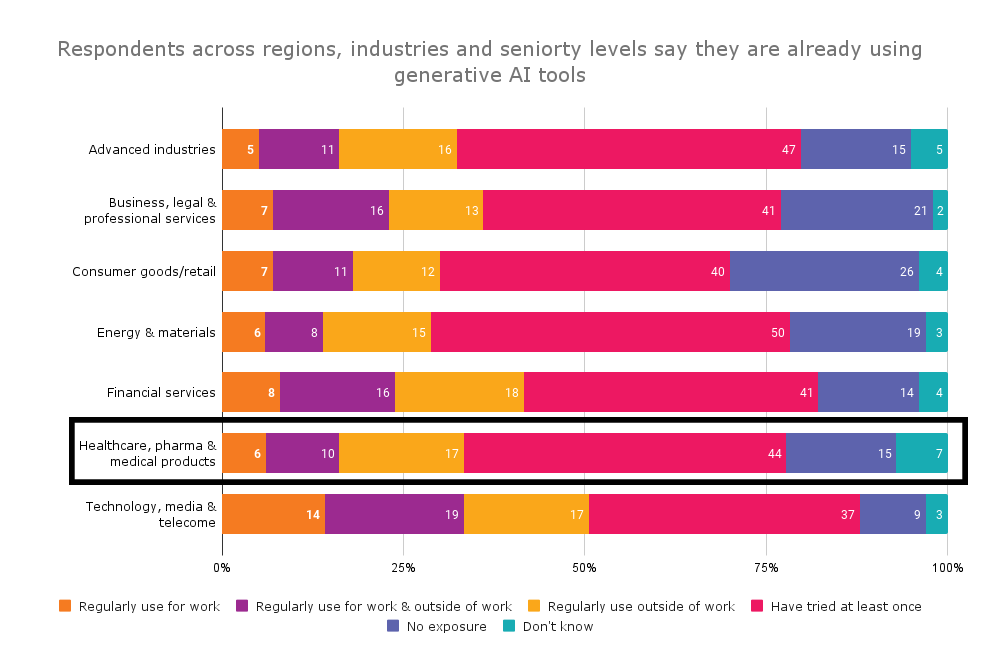
In April 2023, McKinsey & Company conducted its annual survey of global executives. This year’s survey of 1,684 global leaders found 79 percent had some exposure to gen AI, either for work or outside of their profession. As noted in The State of AI in 2023: Generative AI’s Breakout Year, “Less than a year after many of these tools debuted, one-third of our survey respondents say their organizations are using gen AI regularly in at least one business function.”
Among leaders and managers in healthcare, pharma and medical products companies, 85 percent have had some exposure to AI. Nearly a quarter (23 percent) are regularly using AI at work or outside of work and 44 percent have tried it at least once.
Reported exposure to generative AI tools by industry
Big pharma and AI
Contemporaneusly, Reuters reported, “Major drugmakers are using artificial intelligence to find patients for clinical trials quickly, or to reduce the number of people needed to test medicines, both accelerating drug development and potentially saving millions of dollars.”
Reuters reports Amgen, Bayer and Novartis are using AI to “scan billions of public health records, prescription data, medical insurance claims and their internal data to find trial patients – in some cases halving the time it takes to sign them up.” Amgen, for example, has used its AI tool, ATOMIC, to cut the time period for enrolling patients in a mid-stage trial by as much as 50 percent. By 2024, the company expects to use the tool for most studies.
Clinical trials: The state of technology adoption
Amidst all the buzz about AI, there is some evidence that technology adoption in clinical trials is beginning to plateau.
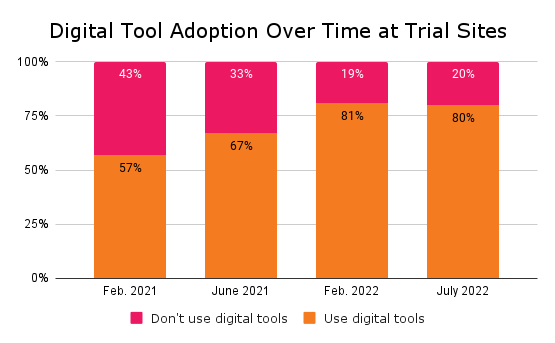
Undoubtedly, the COVID-19 pandemic accelerated technology adoption in clinical trials. In December 2020, when DT Consulting initiated its first Clinical Trial Digital Tracker, 43 percent of surveyed sites used no digital tools to plan or execute trials. Fast forward to 2023 and only 20 percent of sites remain digital-free.
Likewise, the use of decentralized clinical trials (DCT) increased during the pandemic. According to GlobalData analysis, in 2022 about 1,300 drug clinical trials with DCT components started, a 28 percent increase from 2021 and a 93 percent jump from 2020.
SMART on FHIR also is helping to boost technology use in clinical trials. As OpenClinica’s CEO Cal Collins wrote in a recent blog,
SMART on FHIR is a technology framework for apps to integrate with electronic health record systems (EHRs) in a way that is reusable, platform independent, and, in the US, has been widely adopted and supported by EHR vendors and healthcare providers. It provides a very consistent way to do data integration across the many sites that are usually part of a clinical trial.
SMART on FHIR has become widespread, in part, because the standards are mandated through a set of regulations, the 21st Century Cures Act, the HITECH Act, and the Office of the National Coordinator for Health Information Technology (ONC) certification criteria. You can go in with the expectation that there’s going to be support and availability for doing an integration in that way, even if you haven’t previously worked with the site.
That’s a huge difference from the past – where everything was custom, unique and a special snowflake and integrations seemed unattractive compared to manual data entry.
It’s really exciting that’s starting to change.
For OpenClinica clients, SMART on FHIR means our out-of-the-box implementations now require less than 20 hours of effort.
Given the SMART on FHIR mandate, progress towards interoperability and the fact that 90 percent of American hospitals use certified EHRs, I find it sobering that technology adoption in clinical trials is stagnating.
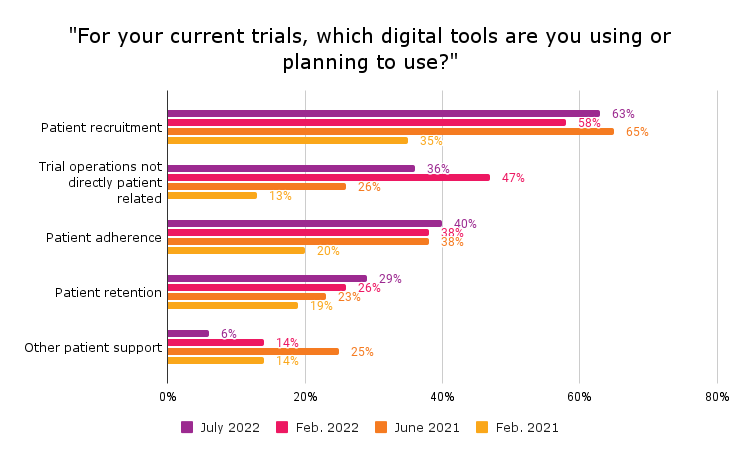
In DT Consulting’s fourth Clinical Trial Digital Tracker, which fielded in June and July 2023, 80 percent of clinical trial sites said they use digital technologies. Rather than increasing, the rate of digital adoption held steady rather than increasing for the first time since the tracker launched in 2020. Among the 80 percent using digital tools, 63 percent use them for patient recruitment, 40 percent for patient adherence, 36 percent to support non-patient-facing clinical trial processes and 29 percent for patient retention.
The percentage of clinical trial sites using digital tools is plateauing
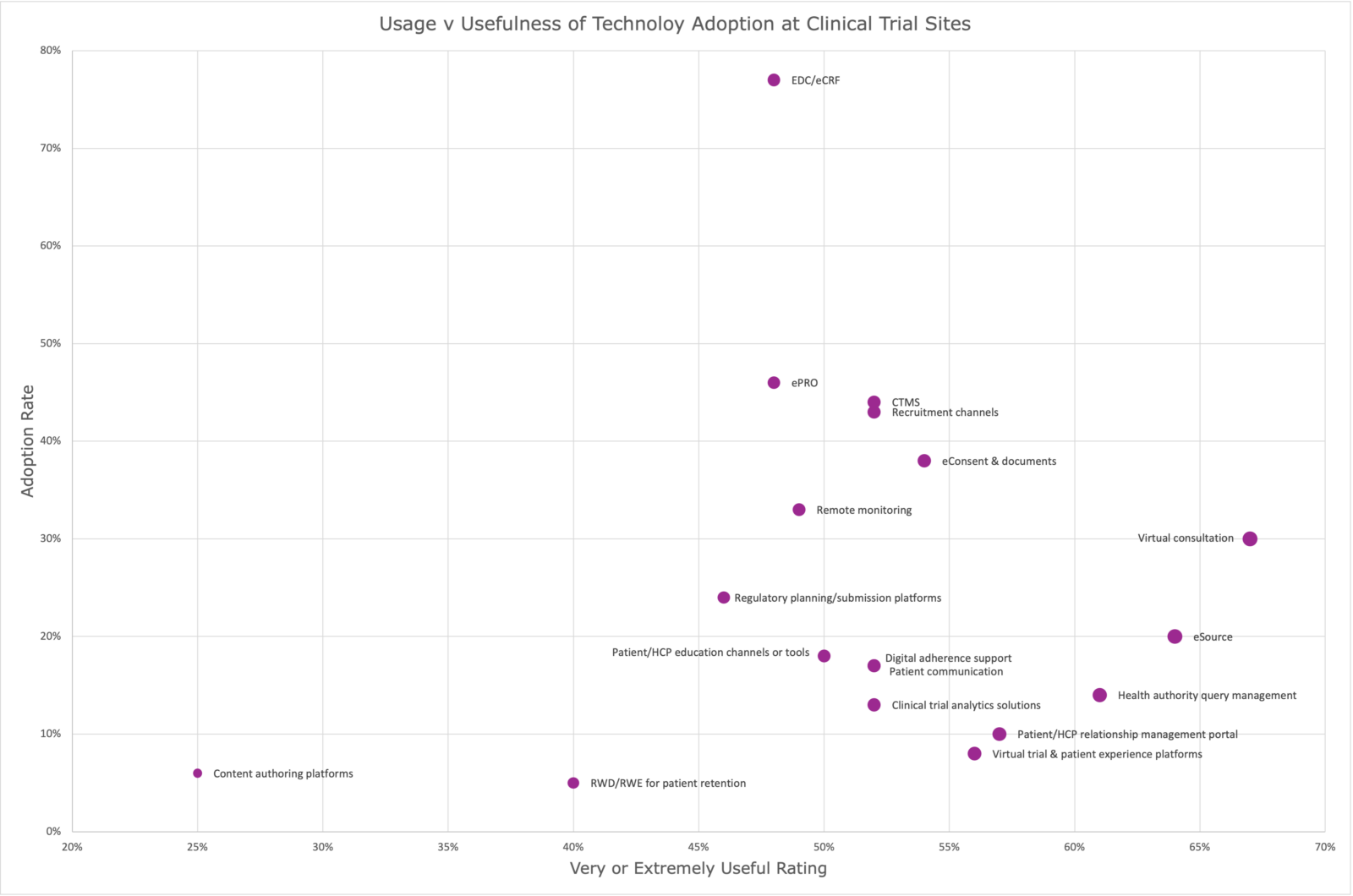
At the same time, we see in the chart below nearly 80 percent of clinical trials use an Electronic Data Capture (EDC) system. About 45 percent of sites use ePRO, less than 40 percent use eConsent and just shy of 20 percent use eSource, a tool that two-thirds of surveyed sites say are very or extremely useful.
Clinical trials adoption of digital tools
As one who has worked in the clinical trials industry for 15 years and witnessed first-hand the growth of EDC adoption from rare to quite common, I recommend these three simple ways to increase technology adoption in clinical trials:
1. Pilot a point solution.
Targeted technology solutions such as ePRO and eConsent are easily deployed, enabling clinical trials to start small and then expand as users’ comfort with change and technology increases.
2. Communicate early with the FDA.
Leverage the FDA’s support of new technology. Talk to the FDA early in the process to boost confidence that the technology will be accepted.
3. Align the technology with the study.
For studies such as a retrospective chart review – in other words, a large abstraction exercise from EHRs – OpenClinica uses our technology to quickly identify eligible patients in those EHRs and automate source data acquisition, accelerating clinical trials by as much as 80 percent.



