I have worked with OpenClinica for more than 15 years and loved how it has developed. The most important pros are the ease of set[ting] up a new study and the management functions. I cannot think of anything [as a con], have worked with clinical studies my entire professional life and used any tool on the market and OpenClinica has resolved all the issues.”
The words of Krister K, a CTO who reviewed OpenClinica on Capterra, are music to my ears. (For more information about OpenClinica’s Capterra reviews, read a recent blog by our CEO, Cal Collins, by clicking here.) That’s because OpenClinica built Insight™, our dashboards and reporting module that is powerful business intelligence (BI) for clinical trial management, to, in part, help sponsors solve site performance monitoring pain points.
Insight gives sponsors unfettered access to ALL of the clinical and operational data in a study. They can very easily review any variable and any parameter, understand how it is structured, where it lives within the study and then organize it into different visualizations, often only with a point-and-click interface. Only the most sophisticated reports require statisticians and data engineers to use structured query language (SQL).
At a high level, Insight enables sponsors to
- Visualize and analyze all EDC clinical data, from screening and enrollment to adverse event (AE) reporting and serious adverse event (SAE) alerts, primarily through pre-built reports.
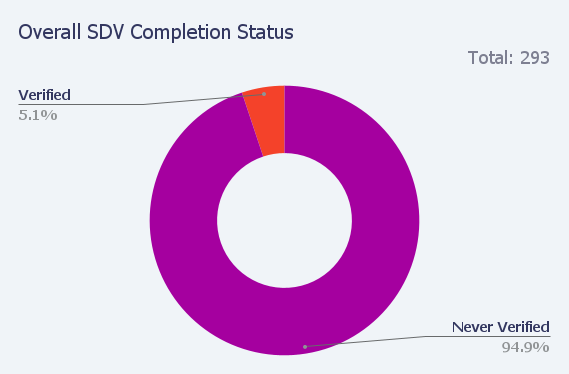
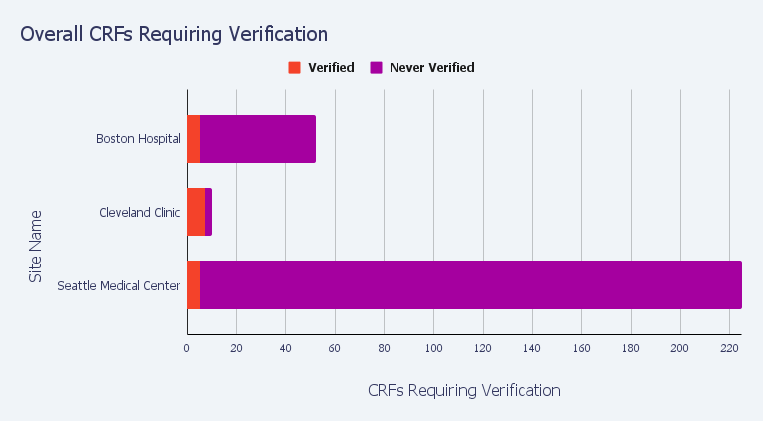
Two examples of our pre-built reports are Overall SDV Completion Status and Overall CRFs Requiring Verification:

There are many more pre-built reports, including:
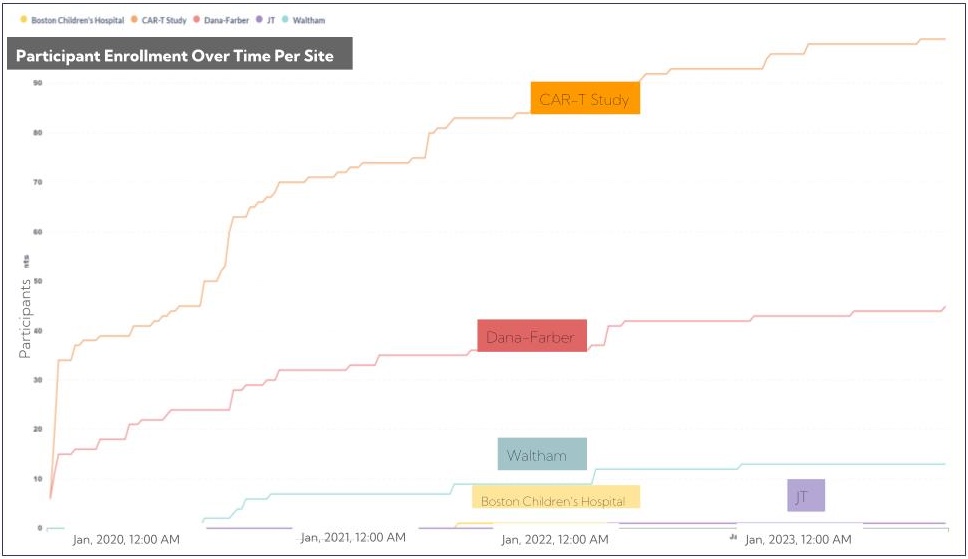
- The cumulative enrollment by site,
- The SDV by event table,
- The missing forms table and
- The query age by site.
2. Easily build customized reports and dashboards to meet unique trial needs.
3. Easily share reports with sites and team members by effectively managing access to and distribution of the dashboards within Insight.
Manage Studies More Effectively: Four Pain Points for Site Performance Monitoring
At the risk of stating the obvious, sponsors have a vested interest in the success of their clinical trials. That means they need to manage studies more effectively with site performance monitoring.
OpenClinica Insight™ helps sponsors easily solve some of the biggest pain points associated with site performance monitoring, including:
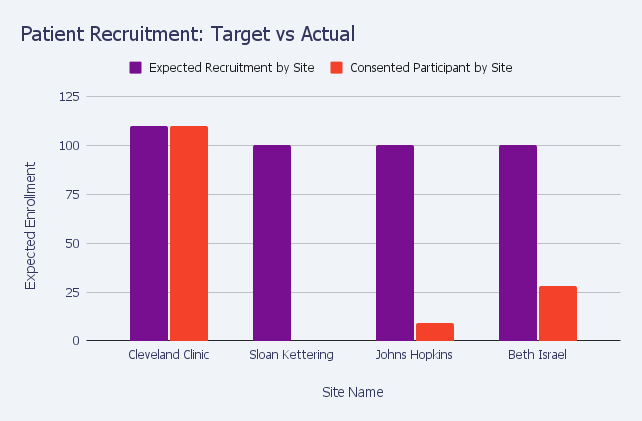
- Patient Recruitment
Sponsors need to know sites are able to recruit patients to their clinical trial. That said, typically each site creates its own marketing and recruitment, meaning patient recruitment is an ad hoc process executed by each site. That’s why sponsors need tools to closely monitor the success of each site by reviewing metrics such as patient accrual, the number of patients screened, the number of failed screens as compared to successfully enrolled patients, and more.
The reports and dashboards in OpenClinica Insight simplify and visualize the status of site patient recruitment activity, enabling sponsors to effectively monitor site performance and, if necessary, prioritize corrective action.


2. Patient Protocol Compliance and Deviations
Once a patient is enrolled in a study, sponsors need to know each patient adhered to the trial protocol. That’s true for all enrolled patients. Imagine there are 500 enrolled patients at 10 different sites who all need to adhere to the same recipe at different times. That’s one colossal logistical challenge.
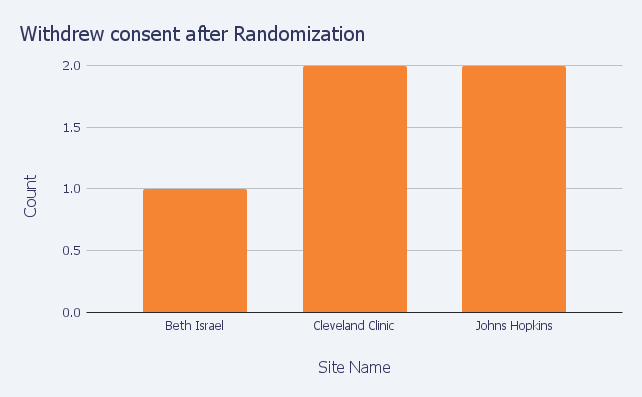
Likewise, if there are protocol deviations, sponsors want to understand why. The context for protocol deviations is critical to determining whether the patient data can still be used in the clinical trial.
With Insight, sponsors can explore key aspects of the protocol such as sites and bottlenecks and improve protocol adherence with ready access to information about dropouts and violations. Insight™ also enables sponsors to receive email alerts about adverse events (AE) and serious adverse events (SAE).
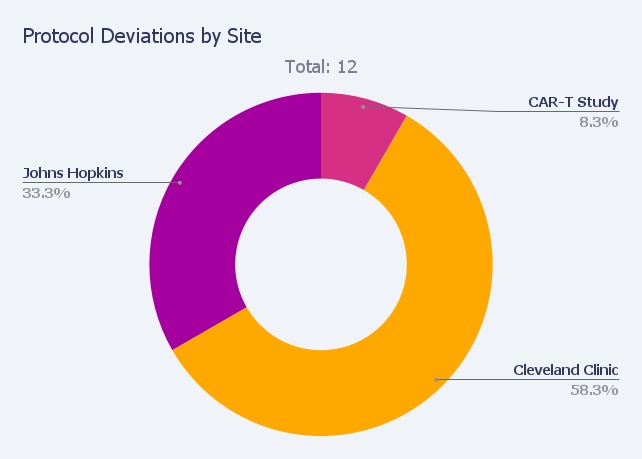
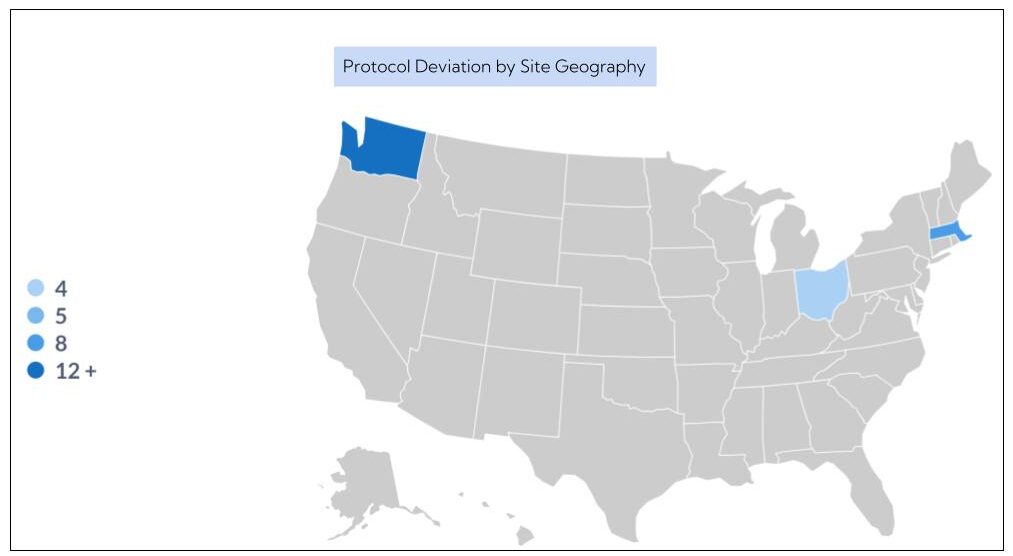
3. Site Assessment and Comparison
Once a clinical trial is underway, sponsors want to readily understand data quality and completeness. To monitor performance, sponsors need to assess data items to ensure they are complete, realistic, conformant, analyzable and sufficiently structured, and all of this information is readily available through Insight.
Again, imagine a clinical trial with 500 patients at 10 sites, five million data points and 10,000 queries. Prioritizing queries related to the trial’s primary and secondary endpoints and patient safety are critical. As shown below, with Insight™, sponsors have ready access to query data and are able to compare site performance on query age, number of AEs, and any number of other measures.

OpenClinica Insight helps sponsors manage studies more effectively by comparing clinical trial site performance.
4. Site Billing
Sponsors use Insight to monitor whether sites have completed services that require payment. Dashboards in Insight allow sponsors to compare site invoices to completed events.
There are 10 top reasons why OpenClinica customers favor Insight for dashboards and reporting, including:
- Automatically tracking enrollment in real time,
- Using dashboards and alerts for more effective monitoring and source data verification, and
- Accessing ALL of your data through easy-to-use, one-click reports.
To view all 10 reasons, click here!
To talk to someone about how Insight can help monitor your site performance, click here!