Integrating electronic health records (EHR) with electronic data capture (EDC) systems can dramatically enhance data accuracy, workflow efficiency, and decision-making in clinical trials. At OpenClinica, we’ve identified nine key advantages of using EHR-to-EDC eSource in clinical trials, including cost savings, scalability and improved site satisfaction. For details, visit the OpenClinica Unite™ product page.
Nine Essential Questions to Ask When Choosing an EHR-to-EDC Solution
Based on our experience supporting clinical trials in the search for an effective EHR-to-EDC solution, we’ve pinpointed nine critical questions to ask prospective vendors. These questions will help you assess the solution’s value, implementation requirements and alignment with your trial needs.
1. How complex is implementation and how long does it typically take?
Implementation should be based on existing industry standards, and configuration-focused rather than relying on custom development, which can add complexity and delay timelines. At OpenClinica, our implementation experience has shown that only 15-20 hours are typically required for configuration at the hospital administrative level. Important points to ask about:
- Use of Standards: If your integration is based on widely adopted healthcare standards (especially Fast Healthcare Interoperability Resources, or FHIR) and pre-integrated with EHR vendors, it should be easy to implement. If not, it will be slow and expensive.
- Configuration vs. Customization: Will the implementation involve primarily configuration or does it require custom development?
- Mapping Processes: Mapping is an important component of the implementation. It’s essential to understand how the data in the EHR gets mapped to its end destination, whether that’s an EDC system or somewhere else. Your vendor should have most common data fields pre-mapped.
- Templates and Rapid Assessment: Does the vendor offer templates or processes for quick assessment of protocol requirements versus available EHR data?
- Future Reusability: How adaptable is the technology for future studies, adding long-term value to your investment?
- Vendor Engagement with Sites: Having a proactive, experienced vendor who takes the lead is crucial for EHR-to-EDC integration success. With site and hospital IT teams often stretched thin by limited bandwidth and lengthy project queues, the right vendor will step in to streamline implementation, minimize the effort required from IT staff, ensure security compliance and deliver clear, actionable guidance.
- Security and Risk: Sites want to ensure any integrations minimize risk to their systems and data. SOC2 certification, app validation by EHR vendor and robust HIPAA and security protections are a must.
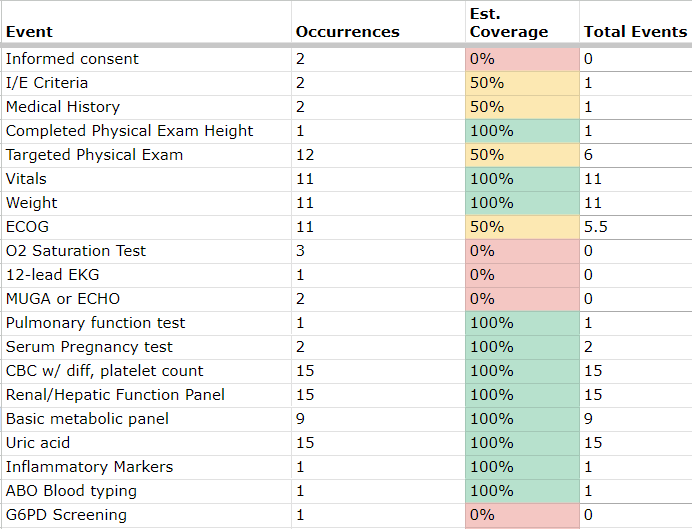
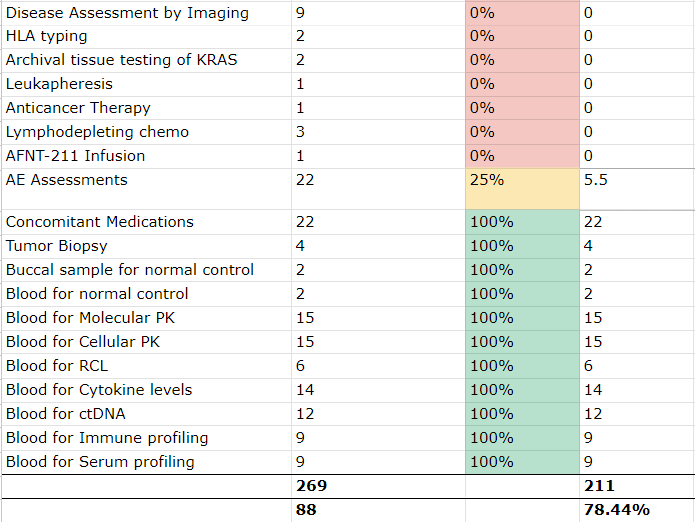
Mapping is an important component of EHR-to-EDC eSource implementation. This mapping example illustrates areas with partial coverage that require deeper analysis.
2. What standards and approaches are used?
To streamline integration, especially across varied site configurations, a strong EHR-to-EDC eSource partner will build on widely recognized standards and have support from major EHR vendors. Here’s what to explore:
- Connection to Data Repositories: How does the tool connect to the EDC system or any resulting data repository?
- User Experience: Does the eSource solution align with existing site workflows? Is it intuitive for end users, or will it require substantial workflow adjustments?
- EHR Launch Capability: Can the solution be launched directly from within the EHR, or does it require a separate interface for the site to learn and manage?
3. How will data be structured and filtered for the sponsor or CRO?
Understanding the structure and flow of data is crucial for trial success. When assessing data delivery, consider:
- Data Presentation: How will data be formatted and presented to sponsors, CROs or other stakeholders?
- Filtering Levels: What levels of filtering are applied to the data?
- Impact on Usability: Will the data format enhance usability, facilitate analysis and streamline trial management?
4. What results have been achieved with other clinical trial clients?
Real-world experience is crucial for understanding the solution’s practicality and capabilities. Ask the vendor:
- Client Case Studies: Can they provide concrete results, case studies and referrals?
- Operational Impact: What efficiencies or time savings have been documented in other trials?
- Examples of Success in Complex Trials: For example, OpenClinica Unite™ was used in the I-SPY-COVID-19 platform trial, achieving a 61% time savings over manual data abstraction. For details, download the case study here.
5. How does the solution integrate with existing user workflows?
A well-designed EHR-to-EDC integration solution should enhance, rather than disrupt, existing workflows for clinical research staff. Understanding workflow compatibility is essential to a successful implementation and ongoing adoption. Consider asking:
- Workflow Alignment: How well does the solution align with the current processes and workflows of site staff?
- Ease of Use: Is the interface intuitive, minimizing the learning curve for users?
- Data Entry Reduction: To what extent does the solution eliminate or reduce redundant data entry, enabling staff to focus on higher-value tasks?
- Real-time Accessibility: Can data be accessed and transferred in real time, or does it require manual steps at specific intervals?
- Feedback from Existing Users: what feedback has the vendor received form current users on workflow integration and ease of use?
6. Does the vendor have an established network or data pipelines that can be leveraged?
A vendor with an existing network of sites and data pipelines can significantly reduce implementation time. Key points to verify:
- Existing Partnerships: Does the vendor have established relationships with major health systems?
- Implementation Efficiency: Can a new client leverage an existing network or data pipelines to simplify setup?
- Reputation and Reach: How broad is the vendor’s footprint? OpenClinica Unite™ is deployed at over 450 clinical trial sites, including top-tier institutions like Columbia University Medical Center and MD Anderson Cancer Center.
7. What data privacy and security measures are in place?
Privacy and security are paramount when handling protected health information (PHI). Confirm the following:
- Data Control and Oversight: Does the solution keep data control with clinical research coordinators (CRCs) and principal investigators (PIs)?
- Security Layers: Is the solution equipped with multiple security layers, including part 11 and GCP compliance?
- Compliance Documentation: Can the vendor demonstrate their track record for privacy and security?
- Data Traceability: Does the tool provide data lineage and traceability, meeting regulatory-grade standards?
8. How does the vendor handle training and go-live support?
Training and support protocols are essential for smooth onboarding. Consider these aspects when discussing training with your vendor:
- Training Duration: Can training and go-live support be completed in under an hour?
- Ease of Onboarding: How quickly can users begin automatically transferring data from the EHR to the EDC?
- Ongoing Support: Will the vendor provide continuous support post-launch if issues arise?
9. How will the vendor support trial sites post-implementation?
The most credible EHR to EDC technology partners have a clear plan for ongoing support across the trial network. Done right, there will be little need for support and maintenance, but if issues arise, you need to know your partner will be there! Consider:
- Support Strategy: What is the vendor’s approach to post-implementation support across sites?
- Dedicated Support Team: Does the vendor offer a dedicated team to assist with troubleshooting and ongoing support?
- Rapid Issue Resolution: Is there a protocol for fast support if issues arise?
- Proactive Maintenance and Updates: Will the vendor provide proactive maintenance and regular updates to keep the technology optimized?
Ready to experience OpenClinica’s EHR-to-EDC solution? Request a demo to see firsthand how Unite can enhance your clinical trials.

