Last week, OpenClinica published a blog by our co-founder and chief operating officer, Ben Baumann, Meet OpenClinica’s Solutions Consultants.
ICYMI, Ben introduced some of my colleagues, Caitlyn, Julianne, Leah, and me. He also discussed the work solutions consultants do to help our clients manage studies more effectively, including an engagement I’m particularly proud of – a massive data migration project for the Imperial College of London (Imperial).
In short, we migrated 14 of Imperial’s ongoing studies from their legacy system onto the OpenClinica platform – that is, 14 active studies with 1,700+ patients and 150,000+ forms in 16 months. To learn more about the vital work OpenClinica did with Imperial, visit here!
Feel free to visit Imperial’s website for additional information, here!
OC24: OpenClinica User Conference
OpenClinica hosted its annual user conference, OC24, in June. As Ben wrote in a recent blog:
For two days, OpenClinica’s global community came together to shape the future of clinical trials. Users participating from their desk meant we had attendees from all over the United States and the United Kingdom but also from Kenya, Poland, Argentina, Normandy region of France, France, Belgium, Austria, Switzerland, Germany, Denmark and even Tajikistan.
I had the privilege of leading a session to help OpenClinica users manage studies more effectively, Building Successful Studies: A Proven Framework, a framework that was created by our team of solutions consultants.
Building Successful Studies: A Proven Framework
Let me say at the outset that the recording of my OC24 session is available to OpenClinica customers. Simply email your customer success manager.
For those of you who want the Cliff Notes version of my presentation, read on. It was an action-packed 45 minutes with seven themes:
- Guiding Principles of Study Design: Important considerations when initial study conversations commence
- Study Personas: The people who need to be a part of those conversations
- Core Design: What to think about when you are building the study
- Libraries: When you are ready to make your studies more efficient
- Testing: Ways to ensure your testing is accurate and holistic
- Scoping & Managing Timelines: How to create a project plan and manage to the timelines
- Amendments
Building Successful Studies: Principles of Study Design
In the Guiding Principles of Study Design section, I reviewed the three big Ws: What, Why and Who.
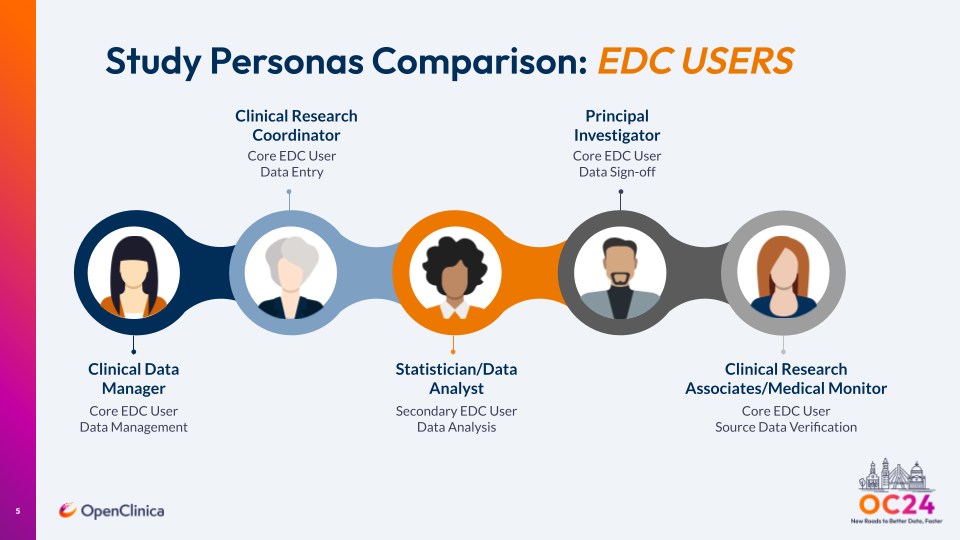
Building Successful Studies: Study Personas
In terms of study personas, there are five primary players in our framework. Four of them – clinical data manager, clinical research coordinator, principal investigator and clinical research associates/medical monitor – are core EDC users – and the fifth is a statistician/data analyst who is a secondary EDC user.
In our experience at OpenClinica, we believe it is important to involve all five of these roles at the beginning of the study. That way, they can provide their feedback on precisely how the data needs to be, which should subsequently simplify the data analysis. They can also offer their perspectives to study planning and implementation processes. We strongly recommend all of these roles/perspectives be included in the study kickoff call.
Building Successful Studies: Core Design
Design with the output in mind. Important considerations are:
- Visit structure
- Item grouping
If, for example, you have a form that is dedicated entirely to vital signs and another exclusively for labs, categorizing these forms ahead of time and ensuring there is minimal overlap, is useful. - Item naming conventions
The more consistent you are with the way you name your variables, the easier it is for your statistician to analyze them. - OID generation
OpenClinica has an algorithm to generate the OIDs (object identifiers). If you use the algorithm to predict them ahead of time, you can get across your logic faster. - Cross-form logic
Connecting data across forms can help prevent errors, such as filling out a Pregnancy form for a male patient. - Calculations
Deciding whether a study prefers calculations to be seen on the form or hidden determines what kind of data types will be built into the form. - Item constraints
Also known as edit checks. Creating different types of edit checks enables a study builder to hone in on the functionalities of the form and use them to ensure things are not missed, e.g., eligibility criteria.
See below for more tips and tricks:

Building Successful Studies: Libraries
There are eight compelling reasons to use an eCRF library, which is a digital repository that contains eCRFs and their associated programming elements:
- Faster results
- Expanded accessibility
- More consistency for users
- Higher quality
- Greater alignment across studies
- Reduced User Acceptance Testing (UAT) time
- Decreased study build time
- Fewer queries
Building Successful Studies: Testing
There are four kinds of testing:
- Unit testing: Testing individual units or components of study design.
- Integration testing: Testing combined modules, two or more – Labs, CTMS, Safety, ePRO, etc.
- System testing: Testing the entire build for compliance with the requirements.
- Interactive UAT (I-UAT): Testing the system functionality against the requirements to deem the system fit for use and therefore accepted.
For more information about testing, contact an OpenClinica solutions consultant or watch my presentation, Building Successful Studies: A Proven Framework.
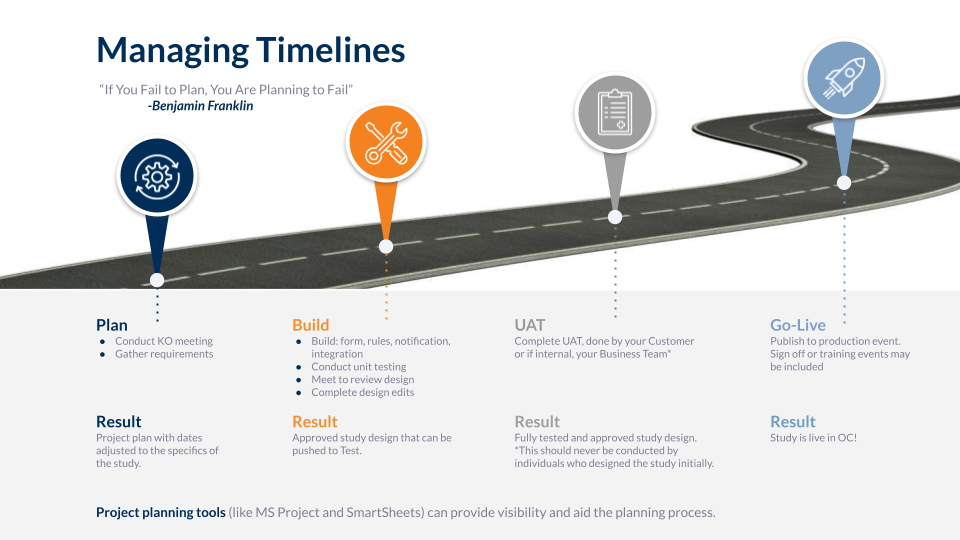
Building Successful Studies: Scoping & Managing Timelines
Building Successful Studies: Amendments
At OpenClinica, we recommend a seven step process for post go-live (PGL) changes, also known as amendments:
- Identify
Compare the protocol differences and identify all changes that affect the build. - Document
Create a running list of the changes that need to be addressed in a working document. - Configure
Create a new version and make the changes in Design and document how all changes were implemented. - Publish to Test
When configuration is stable, publish the configuration to the TEST environment and migrate forms to new version. - UAT
Have end users test the system to make sure all the changes are accounted for and properly configured. - Signoff
When all changes are accounted for, be sure to get a proper sign-off document that says the changes are correct. - Production
Publish to PRODUCTION and then migrate all forms to the new version. Training materials should be updated.
To hear an excerpt from the Q&A section of my presentation, click here. To watch my full presentation, reach out to your Client Success Manager.


