Alzheimer’s disease (AD) has affected around 50 million people worldwide and is projected to reach 152 million by 2060. That same year and every year thereafter, one million Americans are expected to develop Alzheimer’s.
Given the increasing prevalence and the toll it takes on patients, their caregivers and their loved ones, it’s no surprise that there are many clinical trials related to Alzheimer’s disease.
As of May 2024, there were more than 3,300 Alzheimer’s clinical trials. The National Institute on Aging says it’s currently supporting 495 active clinical trials on Alzheimer’s and related dementias.

NIA-Funded Active Alzheimer’s and Related Dementias Clinical Trials and Studies.

These trials reflect diverse drug and mechanistic targets, as well as diversity in the stages of AD/ADRD they address.
The high number of AD and dementia-related clinical trials means there are hundreds of thousands of trial participants. One estimate suggested that there were 429,520 participants in the 1,351 dementia trials between January 2010 and August 2021.
Legally Authorized Representative (LAR) Consent & Alzheimer’s Disease
In the case of participants with Alzheimer’s disease, the informed consent process is more challenging. When a person lacks the capacity to understand the risks and benefits of a research study, informed consent must be obtained from their Legally Authorized Representative (LAR). An LAR is a spouse, guardian, healthcare power of attorney or another individual designated by law depending on the specific situation and jurisdiction. The consent – and, in many trials, subsequent reconsent – of the LAR needs to be documented and associated with the participant’s record, but still maintain integrity as an individual record.
Further, throughout the trial, there usually are LAR-specific assessments that have a cadence, triggering events and data that is different from the primary participant assessment cadence, triggers and data. All data must stay related, but separate.
Beyond Alzheimer’s: Legally Authorized Representative Consent in Clinical Trials
In other cases, participants in some clinical trials must have a study partner. For example, many of Stanford Medicine memory disorders clinical trials require that participants have a study partner. Study partners may be asked to complete questionnaires about the participant, and similar to the consent/reconsent of the LAR, needs to be connected to the participant’s record.
Can you imagine managing all of this documentation with paper files? Too many clinical trials still do. Is it any wonder that clinical research coordinators are burned out!?
There is a Better Way: Automated LAR Workflows Across Consent and Assessments
OpenClinica’s digital workflow for Legally Authorized Representatives solves the informed consent pain point for such clinical trials. In short, the time-saving LAR workflow links the records of the clinical trial participant and their study partner(s), while enabling them to be managed independently. For example, this separation enables the LAR/study partner to be on a study calendar that is distinct, but connected, to the trial participant. Our compelling LAR workflow is sufficiently rare among EDCs such that OpenClinica has clinical trial clients that use our EDC just to deploy this functionality.
eConsent Matrix for Subject and Study Partners
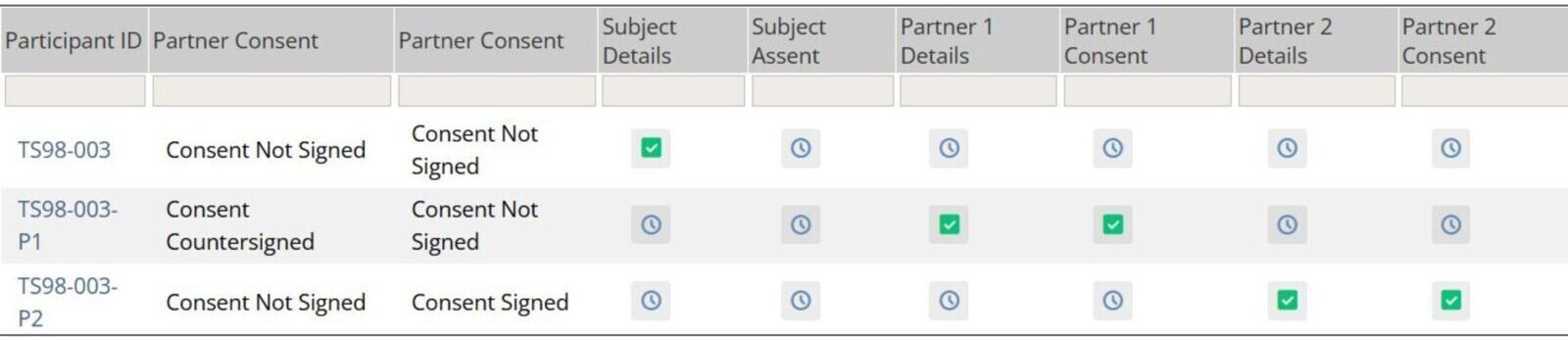
This matrix provides clear line of sight for researchers to easily understand where participants and their study partners are within the consent and re-consent process.
Similarly, the parental consent requirements pose a challenge in adolescent and pediatric clinical trials.
The OpenClinica LAR workflow simplifies informed consent and assessments in clinical trials with children and adolescents, which are estimated to be 39 percent of all reported clinical trials. According to the WHO International Clinical Trials Registry Platform (ICTRP), more than one million infants, children and adolescents are participating in clinical trials, meaning informed consent needs to be obtained and tracked from upwards of two million parents.
World Health Organization. (2024, December). Global Observatory on Health Research and Development. World Health Organization. https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/number-of-trial-registrations-by-year-location-disease-and-phase-of-development
Beyond Alzheimer’s and Adolescent Clinical Trials: LAR in the Wild
In addition to the vast number of clinical trials focused on adolescents and cognitively impaired adults, the OpenClinica LAR workflow can support:
- Critical care of emergency medicine trials: Studies for treatments of conditions like stroke, cardiac arrest, severe COVID and trauma.
- End-of-life or palliative care trials: Research on pain management, quality of life interventions or treatments for terminal illness.
- Psychiatric or behavioral trials: Trials for severe psychiatric conditions like schizophrenia, bipolar disorder or major depressive disorders.
- Development disorders trials: Research on treatments for autism spectrum disorder, down syndrome or cerebral palsy.
- Neonatal trials: Studies on preterm infants, neonatal care protocols or congenital conditions.
- Vulnerable populations: Trials involving individuals in custodial care, such as prisons or long-term care facilities who may have impaired autonomy.
Benefits of OpenClinica’s Automated LAR Workflow for Clinical Trials
In all clinical trials requiring a LAR, the OpenClinica automated LAR workflow ensures:
- Simplified data collection.
- Accurate and separate consent/reconsent/assessment cadence
- Clear link between patient record and LAR record, or records in the case of two parents or multiple LARs.
- Clear separation and privacy of data between patient and LARs.
- Adherence to regulatory compliance for consent and reconsent
Learn More About OpenClinica’s Automated LAR Workflow
For a demo of how OpenClinica’s Legally Authorized Representative workflow solves the pain point of informed consent, reconsent and assessments for vulnerable populations, contact us here.

