What is eSource in Clinical Trials?
Let’s start with a definition of EHR eSource. According to the FDA, eSource is:
Electronic source data are data initially recorded in electronic format… original records and certified copies…of clinical findings, observations, or other activities captured prior to or during a clinical investigation.”
In 2018, the FDA endorsed electronic health record (EHR) eSource writing,
FDA encourages exchange of structured data (e.g., demographics, vital signs, laboratory data) between EHR and EDC systems so that data may be entered once at the point-of-care and used many times without manual re-entry or manual source data verification.”
Just as importantly, the significant momentum leads me to conclude that 2024 will be the year of EHR eSource. Here are 10 reasons why:
10 Reasons to Prioritize EHR eSource in 2024
One: EHR eSource eliminates manual re-entry of EHR data.
Manual data entry is inefficient, burdensome and error-prone, and causes 30-40 percent of trial costs and delays.
Two: EHR adoption is now 98 percent.
Ten years ago, EHR adoption was 30 percent. Today, nearly every health organization is using an EHR.
Three: The 21st Century Cures Act requires an API based on the FHIR standards.
Signed into law in 2016, the 21st Century Cures Act codified the requirement that all healthcare providers must have an Application Programming Interface (API) based on the Fast Healthcare Interoperability (FHIR) standard.
Four: The evolution of FHIR changes everything.
In short, FHIR is simple, uses modern web standards and provides consistent, high quality data across sites. There is a huge community expanding its use while simultaneously improving it. The regulatory and developer support are unmatched. From a technical perspective, FHIR uses JSON, a modern develop-friendly data standard, and it permits flexible, atomic data sharing.
SMART on FHIR is a technology framework for apps to integrate with EHRs in a way that is reusable across sites, platform independent, and, in the US, has been widely adopted and supported by EHR vendors and healthcare providers. It provides a very consistent way to do data integration across the many sites that are usually part of a clinical trial. Equally important, sites and patients retain privacy and access control.
Five: EHR interoperability standards for clinical research are improving.
One organization driving better interoperability standards is the HL7 Vulcan FHIR accelerator, an ambitious new use of the FHIR Accelerator Program that unites a diverse multi-stakeholder group that includes government and regulatory agencies, standards development organizations, academic sites, technology vendors and patients.
Another is the SCDM eSource Implementation Consortium, which is comprised of leading biopharmaceutical firms, academic medical centers, and healthcare technology providers with the vision of enabling a faster digital exchange of clinical research source data from academic medical sites to industry sponsors’ EDC/Clinical Data Management Systems (CDMS) to support faster decision making and improve patient safety. The Consortium seeks to streamline existing data transfer processes to free up site staff to focus on higher value patient touchpoints and bring efficiencies to sites.
Six: The availability of EHR data has increased.
The rapid pace of advancement means EHR data quality, accessibility and completeness are radically better than just a few years ago. A well-regarded EHR certification program ensures consistency and quality.
USCDI v3 Data Elements
Seven: EHR eSource works.
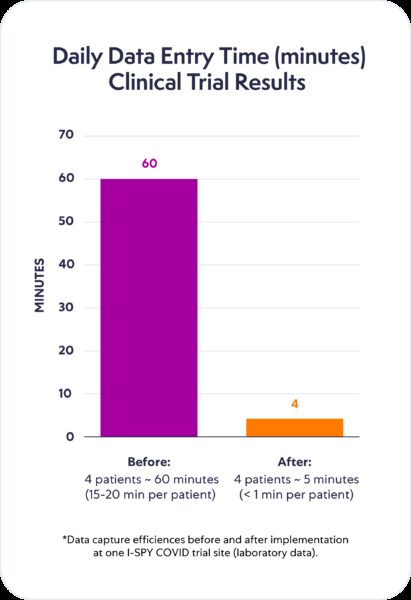
The switch from manual data entry to digital data transfer enabled hours of data collection work on some of the most tedious and I would argue probably some of the most queried data to be completely accurate in just minutes, reducing 60 minutes of manual entry into five minutes of downloading source data. The subsequent monitoring visits went so much quicker, and the number of queries dropped dramatically.”
The use of eSource technology, compared to manual data entry, resulted in:
- A 16-minute per form savings for elapsed data entry time on the primary daily eCRF, a 61 percent reduction compared to manual entry;
- For just 52 patients studied at one site with a 15-day treatment course, 208 hours of clinical research coordinator time was saved;
- The elimination of data errors by the automated, validated EHR-loaded data and the downstream costs associated with cleaning, source data verification (SDV) and validation;
- Low implementation costs, and
- Reusability across sites.
Eight: EHR eSource benefits sponsors and sites.
For sponsors, EHR eSource means:
- Higher quality data that is cleaner and more complete,
- Faster access to study data,
- Cost reductions to the practice of SDV, and
- Stronger partnerships with sites.
For sites, EHR eSource means:
- Reduced research coordinator burden,
- Easier patient participation in research,
- Increased focus on patients, and
- The ability to do more research.
Nine: EHR eSource implementation has been simplified.
At OpenClinica, we encourage organizations to pilot EHR eSource to evaluate first-hand before committing significant resources and changing standard processes and to measure results.
A successful pilot of EHR eSource includes:
- Pick the right pilot site. Start with active study site – live, enrolling, relatively
high volume. - Get site staff onboard. Ensure they are willing to explore capabilities, bring in your tech partner.
- Set clear expectations. Short, regular program management meetings once per week keep everyone aligned.
- Take baseline measurements such as time to enter data manually, time spent on SDV, etc.
- Take ongoing measurements.
- Compare end data with ongoing and baseline.
- Survey site staff.
At OpenClinica, we have learned that not all sites need to adopt EHR eSource for organizations to realize a positive ROI.
Ten: Scaling EHR eSource is straightforward.
A rigorous pilot helps when organizations are ready to scale EHR eSource. So are reusable mappings between the EHR and EDC and the existing site network.
To learn more about why 2024 is expected to be the year of EHR eSource, watch the webinar, A Step-by-Step Approach to EHR eSource in Clinical Trials. If you’re ready to get started, complete our free trial form: Free Trial Request.

